Unstained paraffin sections offer very low contrast and therefore cannot be evaluated microscopically in routine histopathology. It is necessary to apply coloring reagents (mostly chemicals) to stain tissue structures. There are many histochemistry staining techniques that can be applied to examine specific tissue or cell structures. As most of these dyes are water soluble, tissue sections should be rehydrated to remove paraffin (using xylene, alcohol solutions ending in water). Hematoxylin and Eosin (H&E) is the routine staining used to study histopathology changes in tissues and organs from animals in toxicity studies. Hematoxylin is a basic dye that has affinity for acid structures of the cell (mostly nucleic acids of the cell nucleus), and eosin is an acidic dye that binds to cytoplasmic structures of the cell. As a result, H&E stains nuclei in blue and cytoplasms in orange-red. A variant to this staining method is the Hematoxylin–Eosin and Saffron (HE&S) stain. As compared to the H&E method, HE&S stains collagen in yellow-orange, allowing a better highlight of interstitial conjunctive tissue
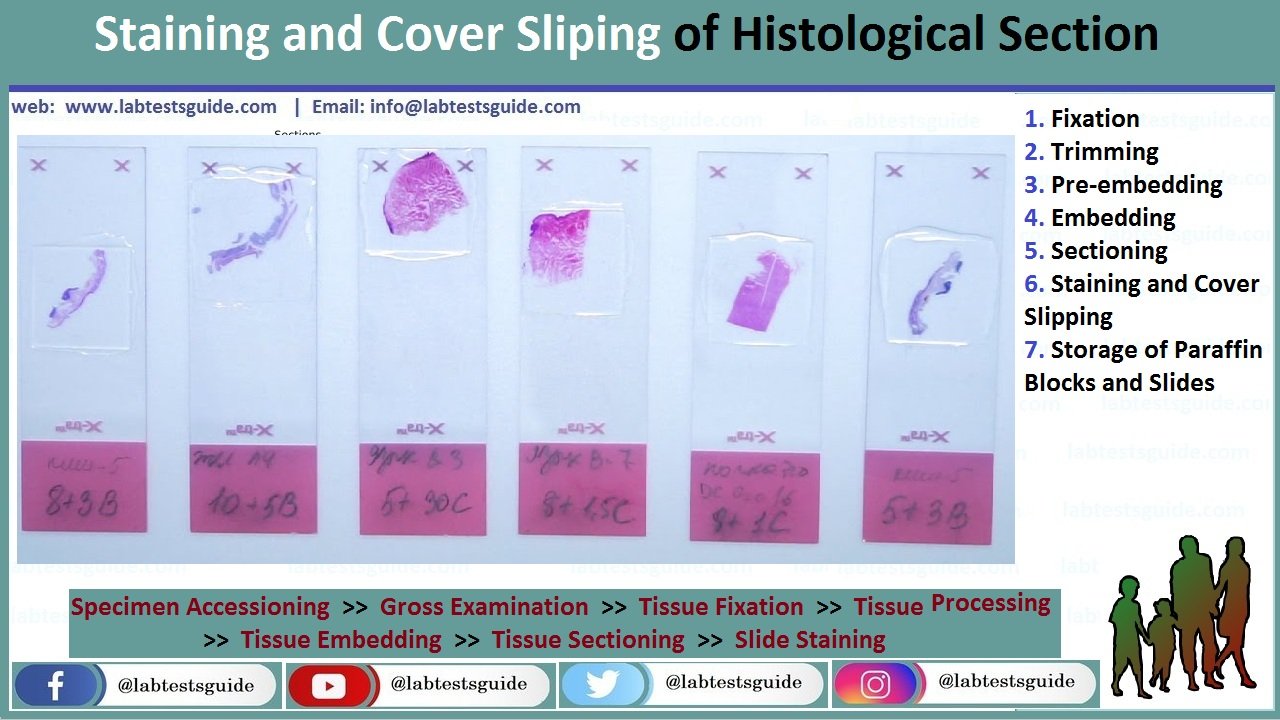
The following protocol describes manual H&E staining technique. It is suitable for small series of slides. This operation is usually automated to allow high-throughput staining of slides.
- Prepare the Harris hematoxylin working solution. Filter the commercial solution through filter paper to remove the metallic precipitant that forms in the solution upon standing.
- Prepare 0.1% aqueous eosin Y working solution. Dissolve 1 g of eosin Y in 1 L of deionised water. Add four drops of HCl to obtain a pH between 4 and 5.
- Add a thymol crystal to prevent molds growth.
- Label and date the solutions. Aqueous eosin solution is stable for at least 2 months at room temperature.
- Prepare the differentiation solution (acid alcohol). Add 1 mL of 37% HCl to 100 mL of 70% ethanol.
- Dewax the paraffin sections in xylene 2 × 5 min each.
- Rehydrate in 100% ethanol 2 × 5 min each.
- Rehydrate in 95% ethanol 2 × 5 min each.
- Wash in running tap water for 3 min.
- Stain for 3–5 min in Harris hematoxylin.
- Wash in running tap water for 3 min.
- Decolorize briefly in acid alcohol for 2 s.
- Wash and blue the sections in running tap water for 3 min.
- Stain for 2–5 min in 0.1% aqueous eosin Y.
- Rinse in tap water for 30 s.
- Dehydrate in 95% ethanol two times for 2 min each.
- Dehydrate in 100% ethanol two times for 2 min each.
- Clear sections in clearing agent two times for 2 min each.
- The slides may remain in clean clearing agent until cover slipping.
- The nuclei will be stained in blue, the cytoplasms and other tissue components in pink-orange. Decreased staining intensity of the sections (after approximately 500 slides) indicates that the staining solutions should be renewed.
After staining, a very thin glass should be placed over the tissue section to protect it and to enhance the optical evaluation of the tissue. This also allows tissue section storage for several years. Cover slipping process consists of gluing the cover slip glass over the tissue section on the microscope slide glass. The mounting medium is usually insoluble in water. Therefore, the tissue should be dehydrated again using solutions of increasing concentrations of alcohol and xylene.
The following protocol describes manual cover slip mounting technique. It is suitable for small series of slides:
- Wipe the surface under the slide while keeping the tissue section covered with the clearing agent.
- Apply two or three drops of the mounting medium (e.g., Eukitt®).
- Place a cover slip on the slide and avoid the formation of bubbles. Press gently with forceps to remove any bubble.
- Dry the slides overnight at room temperature on a flat surface within the fume hood.
To allow high-throughput slide preparation, this operation is usually automated. These automated machines are commercially available from many suppliers.
Materials:
- Fixation:
- Fixative solution (usually commercially available formalin).
- Phosphate buffer (pH = 6.8).
- Rubber or gloves (see Note 1).
- Protective clothing.
- Eyeglasses and mask.
- Fume hood.
- Containers with appropriate lids (volume is commensurate
with sample size. Large neck plastic containers are preferable
and can be reused). - Labels and permanent ink
- Trimming:
- Fume hood.
- Rubber or gloves
- Protective clothing.
- Eyeglasses and mask.
- Dissecting board (plastic boards are preferred as they can be easily cleaned and autoclaved).
- Blunt ended forceps (serrated forceps may damage small animal tissues).
- Scalpels blades and handle.
- Plastic bags and paper towels.
- Containers for histological specimens, cassettes and permanent abels. Containers and cassettes, should be correctly labeled before starting tissue trimming.
- Pre-embedding
- Disposable plastic cassettes for histology (with appropriate
- lids). For small samples, disposable plastic cassettes for histology with subdivision (Microsette®).
- Foam pads (31 × 25 × 3 mm) can be used to immobilize tissue
samples inside the cassettes. - Commercial absolute ethyl alcohol and 96% ethanol solution.
- 90% and 70% ethanol solutions.
- Paraffin solvent/clearing agent: xylene or substitute (e.g.,
Histosol®, Neoclear®). - Paraffin wax for histology, melting point 56–57°C (e.g.,
Paraplast® Tissue Embedding Media). - Automated Tissue Processor (vacuum or carousel type).
- Embedding
- Tissue embedding station (a machine that integrates melted paraffin dispensers, heated and cooled plates).
- Paraffin wax for histology, melting point 56–57°C (e.g. Paraplast® Tissue Embedding Media).
- Histology stainless steel embedding molds. These are available in different sizes (10 × 10 × 5 mm; 15 × 15 × 5 mm; 24 × 24 × 5 mm; 24 × 30 × 5 mm, etc.).
- Small forceps.
- Sectioning:
- Rotary microtome.
- Tissue water bath with a thermometer. Alternatively a thermostatic warm plate can be used.
- Disposable microtome blades (for routine paraffin sections use wedge-shaped blades).
- Sharps container to discard used blades.
- Fine paint brushes to remove paraffin debris.
- Forceps to handle the ribbons of paraffin sections.
- Clean standard 75 × 25 mm microscope glass slides (other dimension microscope glass slides are commercially available).
- Laboratory oven (set at 37°C).
- Coated glass slides (e.g., Superfrost® or Superfrost Plus®). This is especially recommended when slides are used for immunohistochemistry.
- 0.1% gelatin in water (1 g of gelatin in 1 L of distillated water). This should not be used with Superfrost® or Superfrost Plus® slides and should be reserved for immunohistology sections
- Staining and Cover Slipping:
- Harris hematoxylin (commercial solution, ready to use).
- Eosin Y solution.
- Hydrochloric acid 37%.
- Absolute ethanol.
- Ethanol 96%.
- Clearing agent (xylene or substitute e.g. Histosol®, Neoclear®).
- Staining dishes and Coplin jars suitable for staining.
- Permanent mounting medium (e.g. Eukitt®).
- Glass cover slips (25 × 60 mm).
- Filter paper.
- Ethanol solutions:
- Add 12.5 mL of water to 1 L of commercial 96% ethanol to obtain 95% ethanol.
- Add 408 mL of water to 1 L of commercial 96% ethanol to obtain 70% ethanol.
- Storage of Paraffin Blocks and Slides:
- Paraffin blocks storage cabinets.
- Histological slides storage cabinets.
Realted Posts
Keywords: histopathology procedure pdf ,histopathology procedure ppt ,histopathology procedure manual ,histopathology staining procedure ,fish histopathology procedure ,histopathology lab procedures ,histopathology complete procedure ,histopathology slide preparation procedure ,histopathology lab procedure ,histopathology policy and procedure ,procedure for histopathology ,procedure in histopathology ,histopathology laboratory procedure ,procedure of histopathology ,histopathology tissue processing procedure ,routine histopathology procedure ,histopathology staining procedure pdf ,histopathology test procedure
RELATED POSTS
View all