Once tissue samples are infiltrated by paraffin, they are removed from the cassettes and carefully positioned inside a metal base mold
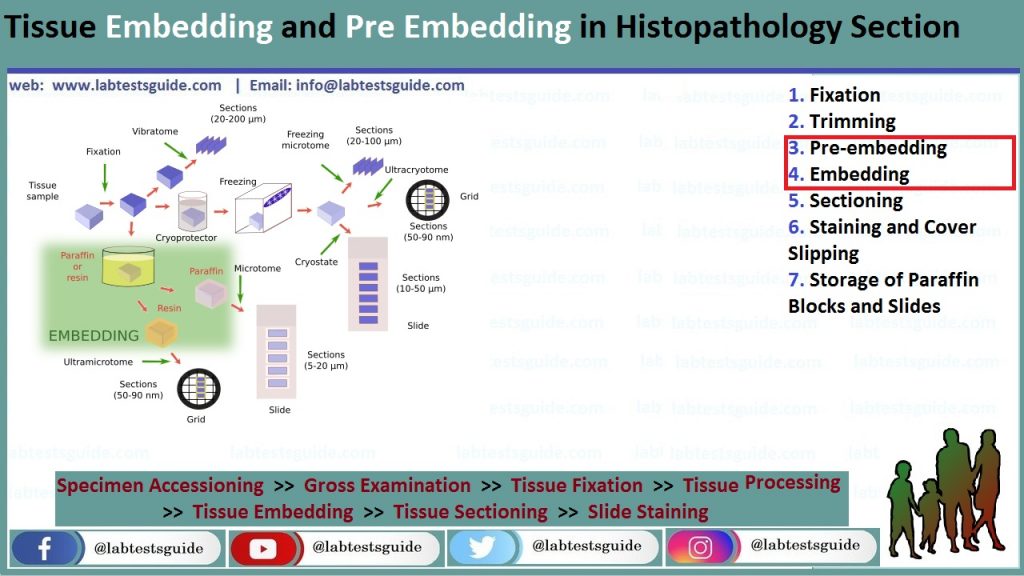
Pre-embedding:
The goal of pre-embedding is to infiltrate tissue samples with paraffin and replace water content of tissue by this wax material . Paraffin is used as a supporting material before sectioning. Histology grade paraffin wax has a melting point around 56 or 57°C, a temperature that does not alter the structures and key morphologic characteristics of tissues, thus allowing adequate microscopic evaluation by the pathologist. At room temperature, paraffin wax offers enough rigidity to allow very thin sections just a few micrometers thick (usually 4 or 5 Mm).
Pre-embedding is a sequential process that consists of dehydration of tissues in increased concentrations of alcohol solutions, then gradual replacement of alcohol by a paraffin solvent. Xylene (or its substitutes; e.g., Histosol®, Neoclear®, and Histoclear®) has the advantage to be miscible in both alcohol and paraffin. As a result, the tissue sample is dehydrated and fully infiltrated byparaffin. This step is generally automated using a variety of vacuum or carousel type tissue processors
When using a tissue processor, the following steps should be followed:
- Check if the baskets and metal cassettes are clean and free of wax.
- Do not pack the tissues too tightly to allow fluid exchange.
- Check if the processor is free of spilt fluids and wax.
- Check if the fluids levels are higher than the specimen containers.
- Select the appropriate protocol and check the clock.
- Prepare 95% and 70% ethanol solutions.
- Dehydrate in a graded series of ethanol:
- Wash in 70% ethanol for 1 h.
- Wash in 95% ethanol for 1 h (two times).
- Wash in 100% ethanol for 1 h (two times).
- Clear with a paraffin solvent (xylene) for 1 h (two times).
- Infiltrate with paraffin for 1 h (two times).
- Tissue sampled are retrieved at the end of the processing program (automates are usually run overnight to start the embedding process in the next morning).
The following is a list of rescue procedures that can be helpful to consider in case the pre-embedding procedure is not completed normally:
- Recovery of tissues that have air-dried because of mechanical or electrical failure of the processor:
- Rehydrate the tissue in Sandison’s solution (absolute alcohol 30 mL, formaldehyde 37% 0.5 mL, and sodium carbonate 0.2 g water up to 100 mL) or Van Cleve and Ross’ solution (trisodium phosphate 0.25 g in 100 mL of water).
- Immerse the tissues in one of these two solutions for 24–72 h (actually most of tissues rehydrate and soften within 4–6 h).
- Process to dehydration and pre-embedding as usual, starting in 70% ethanol.
- Recovery of tissues accidentally returned to fixative following wax infiltration. Discard all contaminated fluids:
- Rinse in 70% ethanol followed by 95% ethanol.
- Rinse in absolute ethanol (two to three times).
- Rinse in xylene (or substitute).
- Carry out the paraffin infiltration three times, 30–60 min each.
- Recovery of tissues accidentally returned to ethanol 70% following wax infiltration:
- Discard all contaminated fluids.
- Rinse in ethanol 95%.
- Rinse in absolute ethanol (two changes).
- Rinse in xylene (or substitute).
- Carry out the paraffin infiltration two to three times, 30–60 min each.
The same steps can be used for manual tissue processing. Melt the paraffin in an oven at 60°C in glass containers. Immerse the specimens into the melted paraffin.
Embedding:
Once tissue samples are infiltrated by paraffin, they are removed from the cassettes and carefully positioned inside a metal base mold.
This step is critical as correct orientation of the tissue is essential for accurate microscopic evaluation. The mold is filled with melted paraffin and then immediately placed on a cooling surface. To trace each tissue specimen, the cassette with permanent tissue and study identification is placed on top of the metal base mold and incorporated in the paraffin block before cooling. In this manner, the cassette will be used as a base of the paraffin block for microtome
sectioning (once the metal base mold is removed)
- Check that the different compartments of the station have the appropriate temperature. Paraffin should be liquid in the paraffin reservoir, work surface should be warm, and cool plate should be cold. Stainless steel molds should be kept warm.
- Remove the cassettes from the last tissue processor bath (normally melted paraffin) and transfer to the warm compartment of the embedding station.
- Transfer one cassette onto the hot plate.
- Snap off the cassette lid and discard it.
- Select a preheated stainless steel mold of the appropriate size. The specimen must not come into contact with the edge of the mold.
- Transfer the mold onto the hot plate.
- Pour melted paraffin from the paraffin dispenser.
- Transfer the paraffin-infiltrated tissues into the mold.
- Using heated forceps, orientate the tissue inside the mold to obtain the desired position in relation with the cutting axis; the specimen surface in contact with the base of the mold being the one that will be on the slide after sectioning.
- Center the specimen in the mold ensuring that paraffin entirely surrounds the edge of the tissue.
- Carefully transfer the mold onto the cool plate. Allow a few seconds to paraffin to turn white (this means that paraffin returned to solid phase). During cooling, the paraffin will
shrink (up to 15% of its initial volume); this compression will be fully recovered later after sectioning. - Make sure that the specimen does not move during this step and still keep its desired orientation. If not, put the mold back onto the warm work surface until the whole paraffin liquefies then start again from step 9. Immediately place the base of the original cassette on top of the mold. Incorporation of the cassette in the paraffin block before cooling allows tracing the specimen identification and uses the cassette as a holder during sectioning.
- Carefully fill the mold with paraffin to above the upper edge of the cassette.
- Carefully transfer the mold and cassette onto the cool plate and allow time (at least 15 min) until the paraffin has hardened.
- Snap off the mold.
- Bring the paraffin blocks together.
- Store the paraffin blocks at room temperature until sectioning
Materials:
- Fixation:
- Fixative solution (usually commercially available formalin).
- Phosphate buffer (pH = 6.8).
- Rubber or gloves (see Note 1).
- Protective clothing.
- Eyeglasses and mask.
- Fume hood.
- Containers with appropriate lids (volume is commensurate
with sample size. Large neck plastic containers are preferable
and can be reused). - Labels and permanent ink
- Trimming:
- Fume hood.
- Rubber or gloves
- Protective clothing.
- Eyeglasses and mask.
- Dissecting board (plastic boards are preferred as they can be easily cleaned and autoclaved).
- Blunt ended forceps (serrated forceps may damage small animal tissues).
- Scalpels blades and handle.
- Plastic bags and paper towels.
- Containers for histological specimens, cassettes and permanent abels. Containers and cassettes, should be correctly labeled before starting tissue trimming.
- Pre-embedding
- Disposable plastic cassettes for histology (with appropriate
- lids). For small samples, disposable plastic cassettes for histology with subdivision (Microsette®).
- Foam pads (31 × 25 × 3 mm) can be used to immobilize tissue
samples inside the cassettes. - Commercial absolute ethyl alcohol and 96% ethanol solution.
- 90% and 70% ethanol solutions.
- Paraffin solvent/clearing agent: xylene or substitute (e.g.,
Histosol®, Neoclear®). - Paraffin wax for histology, melting point 56–57°C (e.g.,
Paraplast® Tissue Embedding Media). - Automated Tissue Processor (vacuum or carousel type).
- Embedding
- Tissue embedding station (a machine that integrates melted paraffin dispensers, heated and cooled plates).
- Paraffin wax for histology, melting point 56–57°C (e.g. Paraplast® Tissue Embedding Media).
- Histology stainless steel embedding molds. These are available in different sizes (10 × 10 × 5 mm; 15 × 15 × 5 mm; 24 × 24 × 5 mm; 24 × 30 × 5 mm, etc.).
- Small forceps.
- Sectioning:
- Rotary microtome.
- Tissue water bath with a thermometer. Alternatively a thermostatic warm plate can be used.
- Disposable microtome blades (for routine paraffin sections use wedge-shaped blades).
- Sharps container to discard used blades.
- Fine paint brushes to remove paraffin debris.
- Forceps to handle the ribbons of paraffin sections.
- Clean standard 75 × 25 mm microscope glass slides (other dimension microscope glass slides are commercially available).
- Laboratory oven (set at 37°C).
- Coated glass slides (e.g., Superfrost® or Superfrost Plus®). This is especially recommended when slides are used for immunohistochemistry.
- 0.1% gelatin in water (1 g of gelatin in 1 L of distillated water). This should not be used with Superfrost® or Superfrost Plus® slides and should be reserved for immunohistology sections
- Staining and Cover Slipping:
- Harris hematoxylin (commercial solution, ready to use).
- Eosin Y solution.
- Hydrochloric acid 37%.
- Absolute ethanol.
- Ethanol 96%.
- Clearing agent (xylene or substitute e.g. Histosol®, Neoclear®).
- Staining dishes and Coplin jars suitable for staining.
- Permanent mounting medium (e.g. Eukitt®).
- Glass cover slips (25 × 60 mm).
- Filter paper.
- Ethanol solutions:
- Add 12.5 mL of water to 1 L of commercial 96% ethanol to obtain 95% ethanol.
- Add 408 mL of water to 1 L of commercial 96% ethanol to obtain 70% ethanol.
- Storage of Paraffin Blocks and Slides:
- Paraffin blocks storage cabinets.
- Histological slides storage cabinets.
Realted Posts
Keywords: histopathology procedure pdf ,histopathology procedure ppt ,histopathology procedure manual ,histopathology staining procedure ,fish histopathology procedure ,histopathology lab procedures ,histopathology complete procedure ,histopathology slide preparation procedure ,histopathology lab procedure ,histopathology policy and procedure ,procedure for histopathology ,procedure in histopathology ,histopathology laboratory procedure ,procedure of histopathology ,histopathology tissue processing procedure ,routine histopathology procedure ,histopathology staining procedure pdf ,histopathology test procedure
RELATED POSTS
View all
