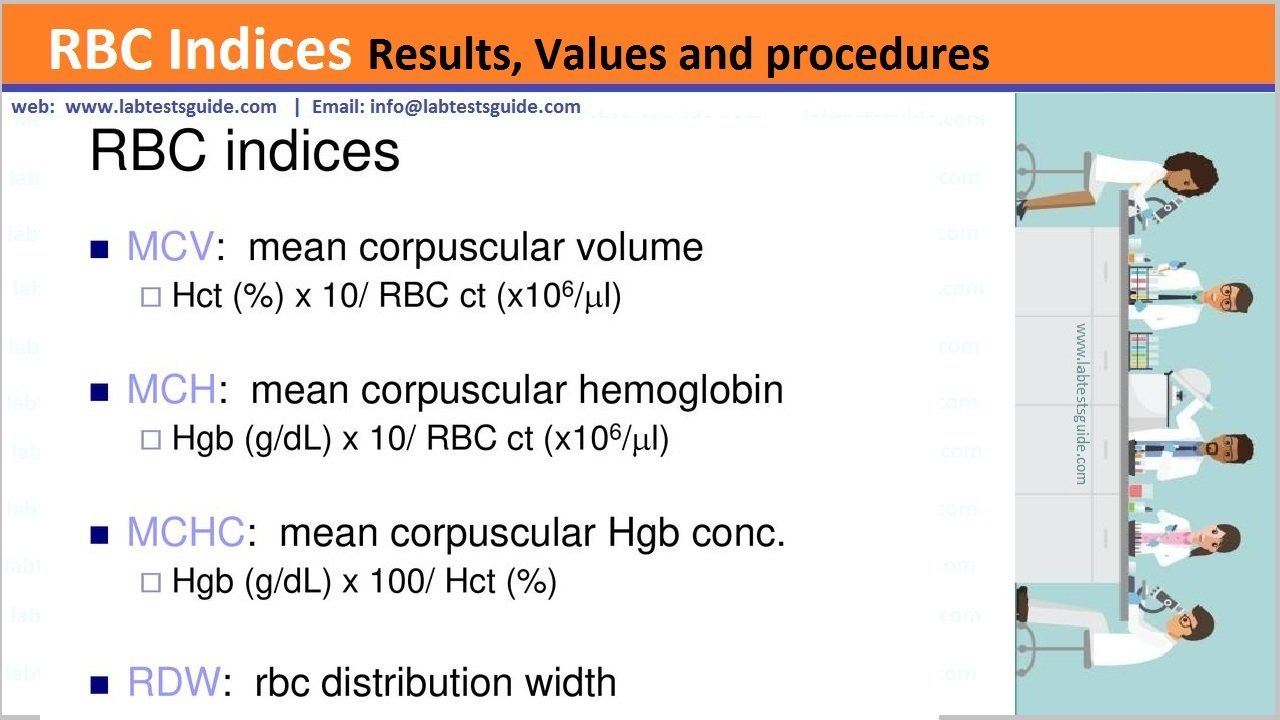
Tissue processing” describes the steps required to take animal or human tissue from fixation to the state where it is completely infiltrated with a suitable histological wax and can be embedded ready for section cutting on the microtome.
Principles of Tissue Processing
- The technique of getting fixed tissue into paraffin is called tissue processing
- Tissue processing is designed to remove all extractable water from the tissue, replacing it with a support medium that provides sufficient rigidity to enable sectioning of the tissue without damage or distortion
Fick’s Law
- To equalize concentrations inside and outside blocks of tissue this depends on Fick’s Law:
- the rate of solution diffusion through tissues is proportional to the concentration gradient (the difference between the concentrations of the fluids inside and outside the tissue) as a multiple of temperature dependant constants for specific substances.
Factors influencing tissue processing
- Agitation
- Heat
- viscosity
- Vacuum
Agitation
- Agitation increases the flow of fresh solutions around the tissue.
- Automated processors incorporate vertical or rotary oscillation.
- Efficient agitation may reduce the processing time up to 30%.
Heat
- Heat increases the rate of penetration and fluid exchange.
- Temperatures limited to 45°C can be used
- Temperatures higher than that can cause tissue brittleness.
Viscosity
- Viscosity is the property of resistance to the flow of a fluid.
- The smaller the size of the molecules in the solution, the faster the rate of fluid penetration (low viscosity) and vice versa
- Most of the processing solutions dehydration and clearing have similar viscosities with the exception of cedar wood oil.
- Embedding mediums have varying viscosities.
- Paraffin has a lower viscosity in the fluid (melted) state, enhancing the rapidity of the impregnation.
Vacuum
- Using pressure to increase the rate of infiltration decreases the processing time.
- Vacuum will remove reagents from the tissue but only if they are more volatile than the reagent being replaced.
- Vacuum can also aid in the removal of trapped air in porous tissue. Impregnation time for dense fatty tissue can be greatly reduced with the addition of vacuum during processing
STEPS OF PROCESSING
1. DEHYDRATION
2. CLEARING
3. EMBEDDING
1. DEHYDRATION
- The first stage of processing is the removal of ‘free’ unbound water and aqueous fixatives from the tissue components.
- Many dehydrating reagents are hydrophilic and interact with the water molecules in the tissue by hydrogen bonding.
- Other reagents affect dehydration by repeated dilution of the aqueous tissue fluids.
- Dehydration should be accomplished slowly.
- If the concentration gradient is excessive diffusion currents across the cell membranes may increase the possibility of cell distortion.
- For this reason specimens are processed through a graded series of reagents of increasing concentration.
- Excessive dehydration may cause the tissue to become hard, brittle and shrunken.
- Incomplete dehydration will impair the penetration of the clearing reagents into the tissue leaving the specimen soft and non-receptive to infiltration.
Dilution dehydration
- Most commonly used method.
- Specimens are transferred through increasing concentrations of hydrophilic or water miscible fluids which dilute and eventually replace free water in the tissues.
Chemical dehydration
- Where the dehydrant, acidified dimethoxypropane or diethoxypropane, is hydrolyzed by free water present in tissues to form acetone and methanol in an endothermic reaction
- In the paraffin wax method dehydration usually initiated in 60%-70% ethanol, progressing through 90%-95% ethanol, then two or three changes of absolute ethanol before proceeding to the clearing stage.
- Dehydration is necessary except where tissues are externally supported by an aqueous embedding medium.
- Choice of a dehydrant is determined by the nature of the task, the embedding medium, processing method, and economic factors.
- Dehydrant differ in their capacity to cause tissue shrinkage
- The dehydrant concentration at which processing is initiated depends largely upon the fixative employed.
- Following fixation in alcoholic fixatives such as Carnoy’s fluid dehydration can be initiated in 100% ethanol
- Duration of dehydration should be kept to the minimum consistent with the tissues being processed.
- Tissue blocks 1 µm thick should receive up to 30 minutes in each alcohol,
- Blocks of 5 µm thick require up to 90 minutes or longer in each change.
- Tissues may be held and stored indefinitely in 70% ethanol without harm.

Dehydrating agents
- Ethanol
- Methanol
- Methylated spirit
- Isopropanol
- Normal and tertiary butanols
- Dioxane
- Polyethylene glycols
- Acetone
- 2,2 dimethoxypropane (DMP)
- 2,2 diethoxypropane (DEP)
- Phenol
- Anhydrous Cupper sulfate
- HCL
- Tetrahydrofuran
Additives to dehydrating agents
- Phenol can be added to dehydrating agents as a softening agent for hard tissues such as tendon, nail, dense fibrous tissue and keratin masses.
- Phenol (4%) should be added to each of the 95% ethanol stations.
- Hard tissue can be immersed in a glycerol/alcohol mixture.
- Anhydrous CuSO4 can act both as dehydrating agent as well as indicator of water
- content of last bath of 100% alcohol. Copper sulfate is layered in the final dehydrating bath and covered with a filter paper if there is any water present copper sulfate will turn blue
Universal solvents
- Universal solvents both dehydrate and clear tissues during tissue processing.
- Examples include Dioxane, tertiary butanol and tetrahydrofuran.
- They are no longer used for routine processing due to their hazardous properties and due to their hardening properties for delicate tissues
2. Clearing
- Clearing is the transition/intermediate step between dehydration and embedding.
- Consists of removal of the dehydrant with a substance that will be miscible with both the embedding medium (paraffin) and dehydrating agent.
- Most clearing agents are hydrocarbons with high refractive indices (approaching that of dehydrated fixed tissue protein) and, on immersion, anhydrous tissues are rendered transparent or clear similar to protein so they are termed as ‘clearing agent’.
Criterion for selection of clearing agents
- rapid penetration of tissues
- rapid removal of dehydrating agent
- ease of removal by melted paraffin wax
- minimal tissue damage
- low flammability
- low toxicity
- low cost
Criterion for selection of clearing agents
- Type of tissues processed
- Type of processing and the processor system to be used
- Processing conditions such as temperature, vacuum and pressure
Factors of clearing
Clearing of the tissues depends on
- Boiling point of clearing agent
- Viscosity
- The boiling point of the clearing agent gives an indication of its speed of replacement by melted paraffin wax. Fluids with a low boiling point are generally more readily replaced
- Viscosity influences the speed of penetration of the clearing agent
Transition solvents
- Toluene
- xylene
- Chloroform
- Carbon tetrachloride
- Trichloroethane
- Esters
- n-Butyl acetate
- Amyl acetate, methyl benzoate and methyl salicylate
Xylene
- Flammable and colorless liquid with a characteristic petroleum odour
- Miscible with organic solvents and paraffin wax.
- Over exposure may cause tissue hardness.
- Most commonly used clearing agent in routine histology and is also recyclable.
Toluene
- Same properties as Xylene
- it is less damaging with prolonged immersion of tissue.
- It is more flammable and volatile than xylene.
Chloroform
- Slower in action
- Little brittleness
- Thicker blocks may be up to 1 mm in thickness can be processed
- Tissues placed in chloroform do not become translucent
- non-flammable but highly toxic and releases the toxic gas phosgene
Citrus fruit oils – limonene reagents
- Limonene reagents are extracts from orange and lemon rinds
- they are non-toxic and miscible with water.
- The main disadvantages are that they can cause sensitization and have a strong pungent odor that may cause headaches.
- small mineral deposits such as copper or calcium may dissolve and leach from tissues.
- They are extremely oily and cannot be recycled.
Automated tissue processors
- Tissue processing can be done manually or on automated tissue processers
- Two types of machines can be used
- Carousel type 2. Enclosed pump fluid type
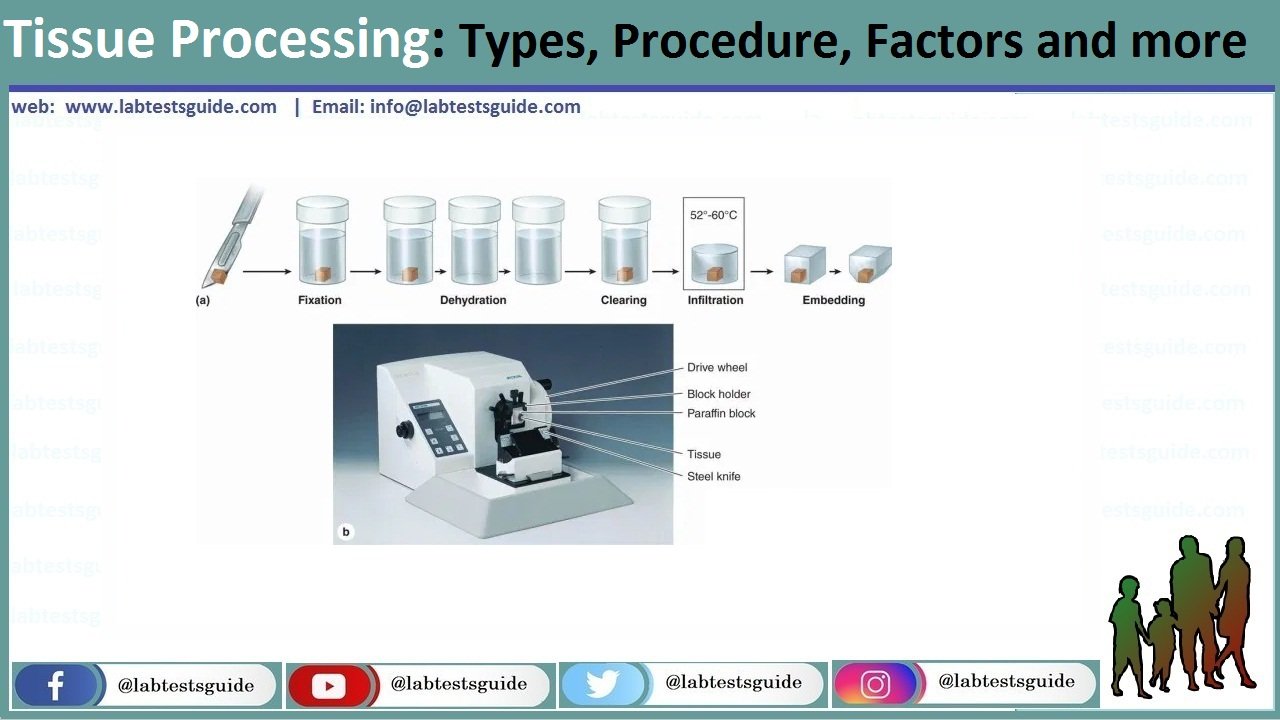
3. Embedding
- It is the process by which tissues are surrounded by a medium such as agar, gelatine, or wax which when solidified will provide sufficient external support during sectioning.
Properties of embedding media
Ideally an infiltrating and embedding medium should be
- soluble in processing fluids
- suitable for sectioning and ribboning
- molten between 30°C and 60°C
- translucent or transparent; colorless
- Stable
- Homogeneous
- capable of flattening after ribboning
- non-toxic
- Odorless
- easy to handle
- inexpensive
Paraffin wax
- most popular embedding medium in histopathology
- It is a mixture of long chained hydrocarbons produced in the cracking of mineral oil.
- Paraffin wax permeates the tissue in liquid form and solidifies rapidly when cooled.
- It has a wide range of melting points ranging from 47 to 64°C which signifies its use in the different climatic
- Heating the paraffin wax to a high temperature alters the properties of the wax.
- It is inexpensive and provides quality sections
- Compatible with most routine and special stains
Paraffin wax additives
- These additives helps to increase hardness
- Substances added to paraffin wax include beeswax, rubber, ceresin, plastic polymers and diethylene glycol distearate.
- Many of these additives had a higher melting point than paraffin wax and make the tissue more brittle.
MODIFIED PARAFFIN WAXES
- The properties of paraffin wax are improved for histological purposes by the inclusion of substances added alone or in combination to the wax:
- improve ribboning: prolong heating of paraffin wax at high temperatures or use micro-crystalline wax
- stearic acid : increase hardness
- spermaceti or phenanthrene : decrease melting point
- 5% ceresin, 0.1-5% beeswax, rubber, asphalt, bayberry wax, or phenanthrene : improve adhesion between specimen and wax (alter crystalline morphology)
- Piccolyte 115, a thermoplastic terpene resin added at the rate of 5%-10% to the infiltrating wax
- Plastic polymers such as polyethylene wax, added to improve adhesion, hardness and plasticity
- Dimethyl sulphoxide (DMSO) added to proprietary blends of plastic polymer paraffin waxes reduces infiltration times and facilitates thin sectioning.
Alternative embedding media
Resin
- Resin is used exclusively as the embedding medium for electron microscopy, ultra-thin sectioning for high resolution and also for undecalcified bone.
Agar
- Agar alone does not provide sufficient support for sectioning tissues.
- Its main use is as a cohesive agent for small friable pieces of tissue after fixation (double embedding)
- Fragments of tissue are embedded in melted agar, allowed to solidify and trimmed for routine processing.
Gelatin
- Gelatin is primarily used in the production of sections of whole organs using the Gough-Wentworth technique and in frozen sectioning.
- It is rarely used.
Celloidin
- The use of celloidin or LVN (low viscosity nitrocellulose) is discouraged because of the special requirements needed to house the processing reagents and the limited use these types of sections have in neuropathology.
- It is rarely used.
Double embedding
- It is the process by which tissues are first embedded or fully infiltrated with a supporting medium such as agar or nitrocellulose, then infiltrated a second time with wax in which they are also embedded.
Embedding tissues in paraffin wax
Requirements for embedding are as follows:
- a supply of clean, filtered paraffin wax held at 2-4°C above its melting point.
- a paraffin dispenser
- a cold plate to rapidly cool the wax.
- a supply of moulds in which to embed the tissues.
- Paraffin wax is dispense automatically from a nozzle into a suitably sized mold.
- The tissue is oriented in the mold, a cassette is attached.
- The mold is placed on a small cooling area to allow the paraffin
Overnight processing schedule
| Reagents | Time | Temperature |
| 10% Formalin | 1 h | 38°C |
| 10% Formalin | 1 h | 38°C |
| 50% Alcohol/formalin | 1 h | 38°C |
| 70% Alcohol | 1 h | 38°C |
| 95% Alcohol | 1 h | 38°C |
| 95% Alcohol | 40 min | 38°C |
| 100% Alcohol | 1 h | 38°C |
| 100% Alcohol | 40 min | 38°C |
| Xylene | 1 h | 38°C |
| Xylene | 30 min | 38°C |
| Paraffin | 30 min | 38°C |
| Paraffin | 30 min | 38°C |
| Paraffin | 30 min | 38°C |
| Paraffin | 30 min | 38°C |
Microtome cutting
The tissue block is attached to microtome and ribbons are cut.
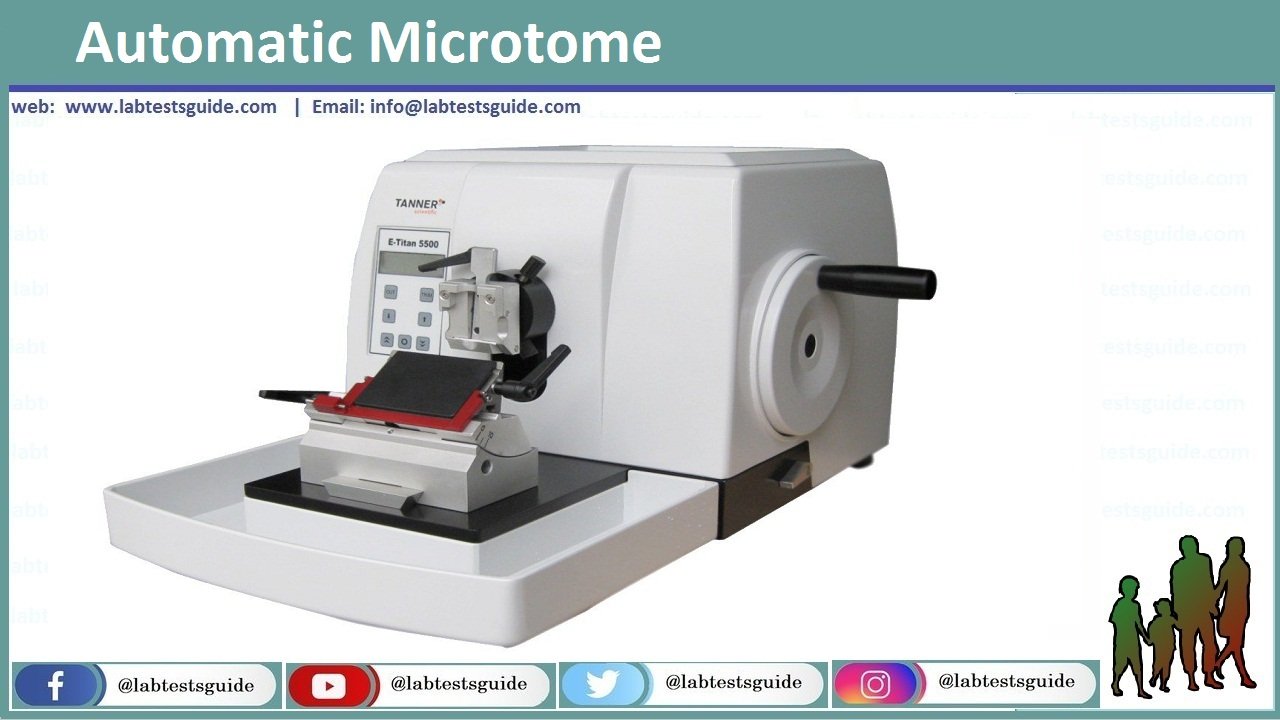
Equipment required for Paraffin section cutting
- Flotation (water) bath
- Slide drying oven or hot plate
- Fine pointed or curved forceps
- Sable or camel haired brush
- Scalpel
- Slide rack
- Clean slides
- Chemical-resistant pencil or pen
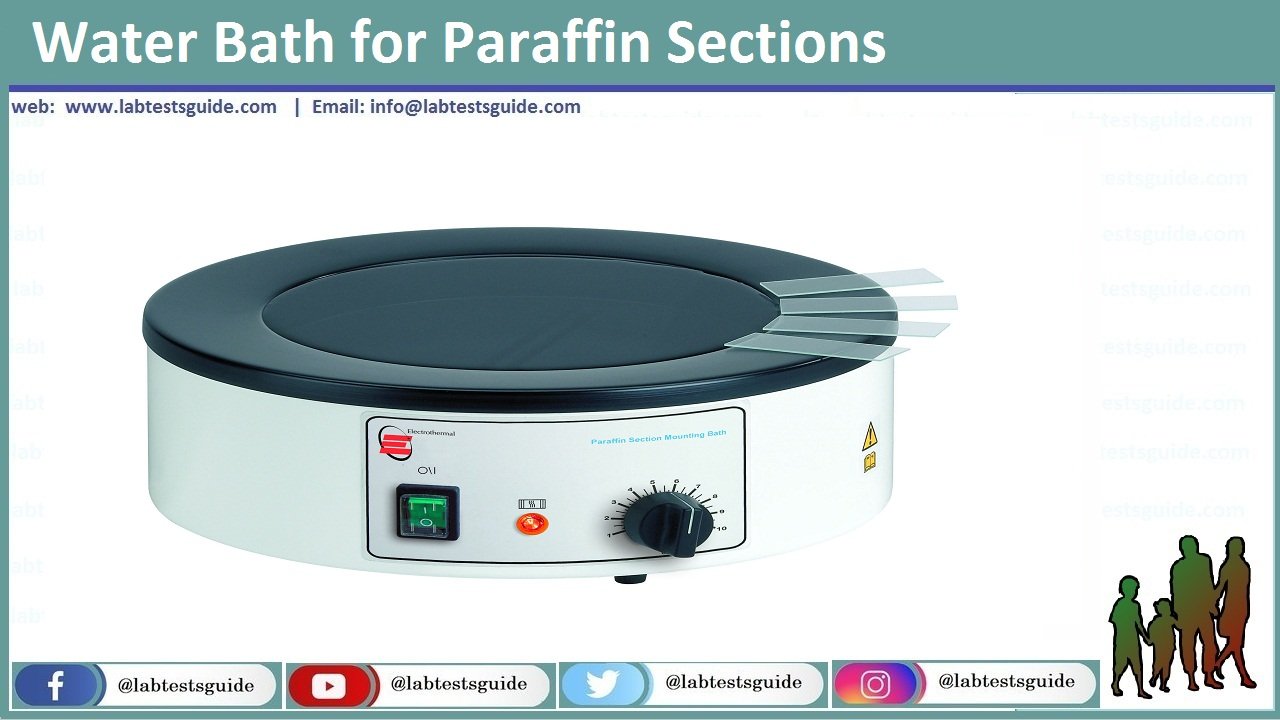
Water Bath
- water bath is used for floating out tissue ribbons after sectioning.
- The trailing end of the ribbon making contact with the water first
- The temperature of the water in the bath should be 10°C below the melting point of the paraffin.
- Alcohol or a small drop of detergent may be added to the water allowing the section to flatten out with greater ease
- 30 seconds are long enough for a ribbon to flatten
Slides
- For normal routine work 76 × 25 mm slides are universally used.
- Those with thickness of 1.0–1.2 mm are preferred
Section adhesives
- Albumen
- gelatin
- Starch
- Poly-L-lysine (PLL)
- 3-aminopropyltriethoxysilane (APES)
Materials:
- Fixation:
- Fixative solution (usually commercially available formalin).
- Phosphate buffer (pH = 6.8).
- Rubber or gloves (see Note 1).
- Protective clothing.
- Eyeglasses and mask.
- Fume hood.
- Containers with appropriate lids (volume is commensurate
with sample size. Large neck plastic containers are preferable
and can be reused). - Labels and permanent ink
- Trimming:
- Fume hood.
- Rubber or gloves
- Protective clothing.
- Eyeglasses and mask.
- Dissecting board (plastic boards are preferred as they can be easily cleaned and autoclaved).
- Blunt ended forceps (serrated forceps may damage small animal tissues).
- Scalpels blades and handle.
- Plastic bags and paper towels.
- Containers for histological specimens, cassettes and permanent abels. Containers and cassettes, should be correctly labeled before starting tissue trimming.
- Pre-embedding
- Disposable plastic cassettes for histology (with appropriate
- lids). For small samples, disposable plastic cassettes for histology with subdivision (Microsette®).
- Foam pads (31 × 25 × 3 mm) can be used to immobilize tissue
samples inside the cassettes. - Commercial absolute ethyl alcohol and 96% ethanol solution.
- 90% and 70% ethanol solutions.
- Paraffin solvent/clearing agent: xylene or substitute (e.g.,
Histosol®, Neoclear®). - Paraffin wax for histology, melting point 56–57°C (e.g.,
Paraplast® Tissue Embedding Media). - Automated Tissue Processor (vacuum or carousel type).
- Embedding
- Tissue embedding station (a machine that integrates melted paraffin dispensers, heated and cooled plates).
- Paraffin wax for histology, melting point 56–57°C (e.g. Paraplast® Tissue Embedding Media).
- Histology stainless steel embedding molds. These are available in different sizes (10 × 10 × 5 mm; 15 × 15 × 5 mm; 24 × 24 × 5 mm; 24 × 30 × 5 mm, etc.).
- Small forceps.
- Sectioning:
- Rotary microtome.
- Tissue water bath with a thermometer. Alternatively a thermostatic warm plate can be used.
- Disposable microtome blades (for routine paraffin sections use wedge-shaped blades).
- Sharps container to discard used blades.
- Fine paint brushes to remove paraffin debris.
- Forceps to handle the ribbons of paraffin sections.
- Clean standard 75 × 25 mm microscope glass slides (other dimension microscope glass slides are commercially available).
- Laboratory oven (set at 37°C).
- Coated glass slides (e.g., Superfrost® or Superfrost Plus®). This is especially recommended when slides are used for immunohistochemistry.
- 0.1% gelatin in water (1 g of gelatin in 1 L of distillated water). This should not be used with Superfrost® or Superfrost Plus® slides and should be reserved for immunohistology sections
- Staining and Cover Slipping:
- Harris hematoxylin (commercial solution, ready to use).
- Eosin Y solution.
- Hydrochloric acid 37%.
- Absolute ethanol.
- Ethanol 96%.
- Clearing agent (xylene or substitute e.g. Histosol®, Neoclear®).
- Staining dishes and Coplin jars suitable for staining.
- Permanent mounting medium (e.g. Eukitt®).
- Glass cover slips (25 × 60 mm).
- Filter paper.
- Ethanol solutions:
- Add 12.5 mL of water to 1 L of commercial 96% ethanol to obtain 95% ethanol.
- Add 408 mL of water to 1 L of commercial 96% ethanol to obtain 70% ethanol.
- Storage of Paraffin Blocks and Slides:
- Paraffin blocks storage cabinets.
- Histological slides storage cabinets.
Realted Posts
Keywords: histopathology procedure pdf ,histopathology procedure ppt ,histopathology procedure manual ,histopathology staining procedure ,fish histopathology procedure ,histopathology lab procedures ,histopathology complete procedure ,histopathology slide preparation procedure ,histopathology lab procedure ,histopathology policy and procedure ,procedure for histopathology ,procedure in histopathology ,histopathology laboratory procedure ,procedure of histopathology ,histopathology tissue processing procedure ,routine histopathology procedure ,histopathology staining procedure pdf ,histopathology test procedure
RELATED POSTS
View all