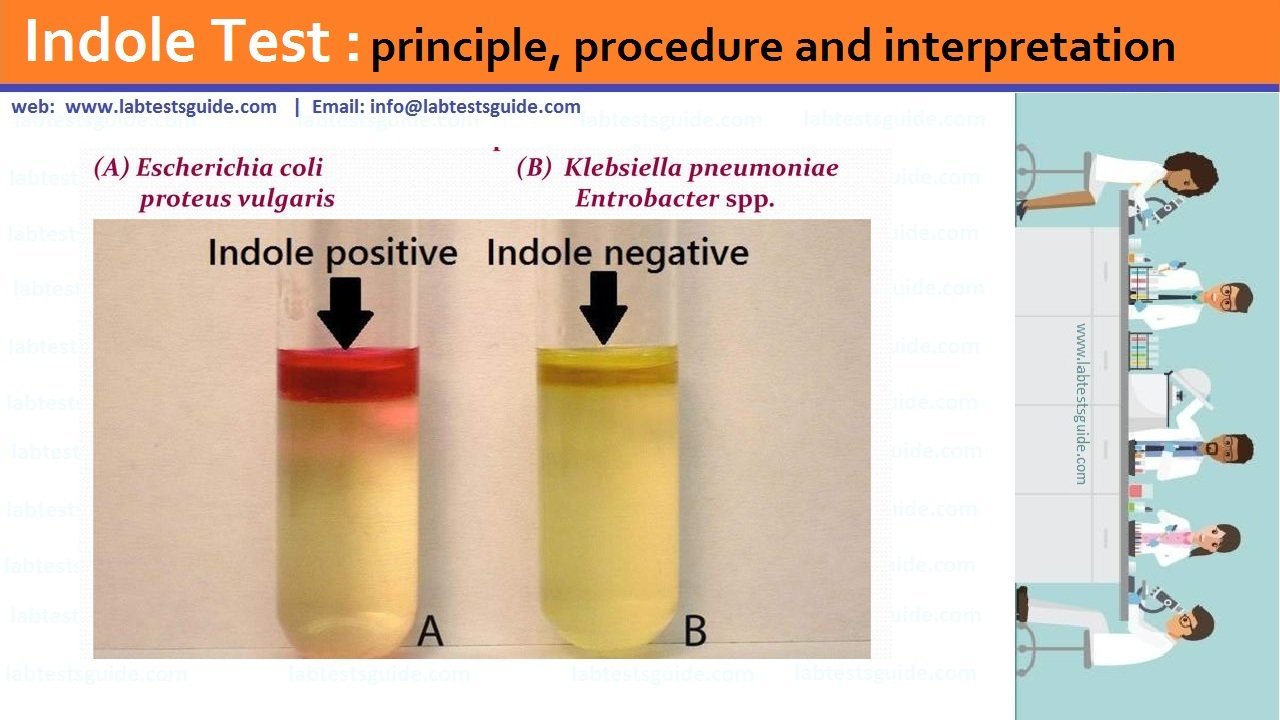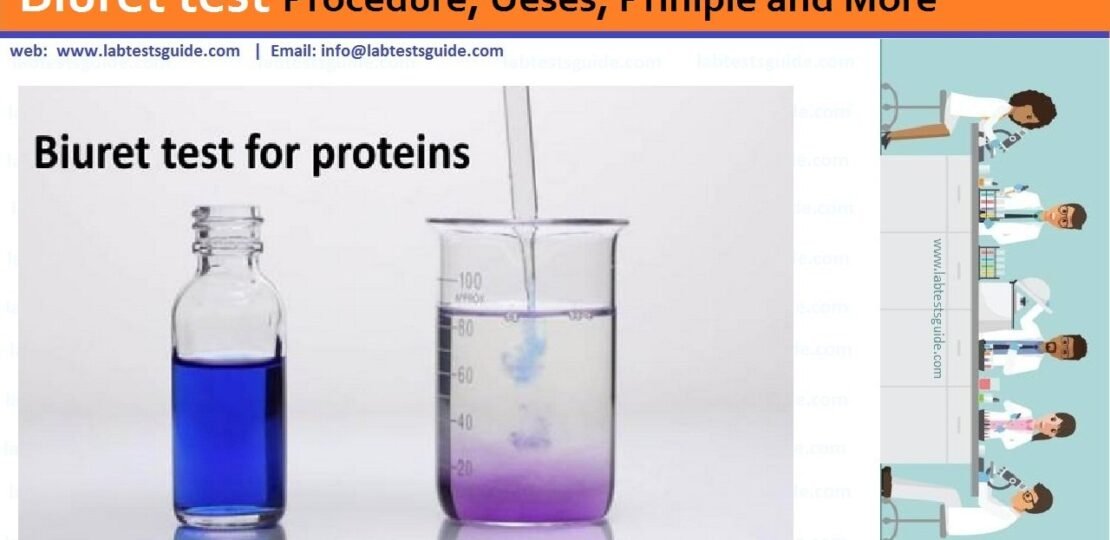
A biuret test is a chemical test that helps verify the presence of proteins in a given sample. To confirm the presence of proteins, it will depend on the color changes.

An indicator that protein is present is when the color changes to violet. Although the test is called biuret, it does not use the chemical biuret; A substance derived from urea. In fact, biuret is not a protein. However, it has the ability to generate a positive result in the biuret test.
Why does biuret turn purple in the presence of proteins?
Biuret is a product of heating urea at approximately 180 degrees Celsius. If it is in alkaline conditions, the biuret is treated with dilute copper sulfate, the color changes to purple. Therefore, it is used to identify proteins in a particular sample, specifically biological fluids.
Performing a Biuret Test
- Requirements:
- 1% alanine and 5% egg white
- Procedure
- Put at least 1 ml of test solutions in the test tube (dry) and another 1 ml in a separate test tube containing 1 mL distilled water as a control.
- Put at least 1 mL of biuret reagent in all test tubes.
- Watch for any changes in color, specifically blue color
What is the biuret reagent?
The Biuret test uses a reagent composed of potassium hydroxide and copper sulfate. Under normal conditions, the color of the biuret reagent is blue. However, change its color to violet if there are peptide bonds. The peptide bond is the chemical bond that holds the amino acids together. There are other alternatives for the biuret reagent and these are copper sulfate and sodium hydroxide. Fehling solutions A and B can also be used.
How to perform the biuret test for protein when using Fehling’s A and B solutions?
- Make sure you prepare a fresh Fehling’s A and B solutions. Keep in mind that A is copper (II) solution while B contains both the solutions of sodium hydroxide and sodium potassium tartrate.
- When testing a food sample, you need to add about 1 cm3 of solutions A and B to the specimen.
- Repeat the aforementioned steps with de-ionized water to have a negative control. For positive control, you should use albumin or egg white.
- Carefully shake the mixture and let it stand there for five minutes.
- Watch for any changes in color.
Performing biuret test using copper sulphate and sodium hydroxide solutions
- Food samples are added with sodium hydroxide solution (1 cm3) and copper (II) sulfate solution (1%).
- Follow the steps mentioned above but this time with de-ionized water to create a negative control. The other one is for positive control using albumin or egg white.
- Shake the mixture and let it stand for about five minutes.
- Carefully observe for any changes in color.
Related Articles:
RELATED POSTS
View all