A microscope is the most expensive and important piece of equipment used in Medical laboratories. Microscopy forms 70–90% of the work.

Microscopy in laboratories
- Malaria
- Trypanosomiasis
- Leishmaniasis
- Onchocerciasis
- Lymphatic filariasis
- Loiasis
- Schistosomiasis
- Amoebiasis
- Giardiasis
- Cryptosporidiosis
- Intestinal Worm Infections
- Paragonimiasis
- Tuberculosis
- Leprosy
- Meningitis
- Gonorrhoea (Male)
- Cholera
- Urinary Tract Infections
- Trichomoniasis
- Vaginal candidiasis
- Red cells in anaemia
- White cell changes
- Skin mycoses
Types of Microscopy
Microscopes used in clinical practice are light microscopes. They are called light microscopes because they use a beam of light to view specimens.
Microscopes that use a beam of electrons (instead of a beam of light) and electromagnets (instead of glass lenses) for focusing are called electron microscopes. These microscopes provide a higher magnification and are used for observing extremely small microorganisms such as viruses.
- Light Microscopy
- Electron Microscopes.
1 Light Microscopy
Brightfield microscopy This is the commonly used type of microscope. In brightfield microscopy the field of view is brightly lit so that organisms and other structures are visible against it because of their different densities. It is mainly used with stained preparations. Differential staining may be used depending on the properties of different structures and organisms.
Darkfield microscopy In darkfield microscopy the field of view is dark and the organisms are illuminated. A special condenser is used which causes light to reflect from the specimen at an angle. It is used for observing bacteria such as treponemes (which cause syphilis) and leptospires (which cause leptospirosis).
Phase-contrast microscopy Phase-contrast microscopy allows the examination of live unstained organisms. For phase-contrast microscopy, special condensers and objectives are used. These alter the phase relationships of the light passing through the object and that passing around it.
Fluorescence microscopy In fluorescence microscopy specimens are stained with fluorochromes/ fluorochrome complexes. Light of high energy or short wavelengths (from halogen lamps or mercury vapour lamps) is then used to excite molecules within the specimen or dye molecules attached to it. These excited molecules emit light of different wavelengths, often of brilliant colours. Auramine differential staining for acid-fast bacilli is one application of the technique; rapid diagnostic kits have been developed using fluorescent antibodies for identifying many pathogens.
Parts of the Microscope
The main parts of the microscope are …
- Eye-pieces
- Microscope tube
- Nosepiece
- Objective
- Mechanical stage
- Condenser
- Coarse
- Fine focusing knobs
- Light source

Eye-pieces
- The specimen is viewed through the eye-piece It has a lens which magnifies the image formed by the objective. The magnifying power of the eye-piece is in the range 5x20x. A movable pointer may be attached to the inside of the eye-piece.
- In binocular microscopes, the two eye-pieces can be moved closer or farther apart to adjust for the distance between the eyes by pulling pushing motion or by moving a knurled ring.
Microscope tube
- The microscope tube is attached on top of the arm. It can be of the monocular or binocular type. It supports the eye-piece on the upper end.
Mechanical tube length
- Mechanical tube length is the distance between the place where the objective is inserted and the top of the draw-tube into which the eyepieces fit.

- In modern microscopes it is not tubular; it contains prisms that bend the light coming up, thus providing a comfortable viewing angle. In a binocular tube, the light is also split and sent to both eye-pieces.
Nose-piece
- The nose-piece is attached under the arm of the microscope tube. The nose-piece houses the objectives and rotates them. The objectives are arranged in sequential order of their magnifying power, from lower to higher. This helps to prevent the immersion oil from getting onto the intermediate objectives.
Objectives
The image of the specimen first passes through the objective. Objectives with magnifying powers 4x, 10x, 40x and 100x are commonly used. The magnifying power is marked on the lens and is usually colour-coded for easy identification
The 100x objective is for oil immersion.
The numerical aperture (NA) is the measure of light-gathering power of a lens. The NA corresponding to the various magnifying powers of the objective is:
| Magnification | Numerical aperture |
| 10x | 0.25 |
| 40x | 0.65 |
| 100x | 1.25 |
A high NA indicates a high resolving power and thus useful magnification
To provide the best image at high magnification, immersion oil is placed between the slide and the oil immersion objective (100x). Unlike air, immersion oil has the same refractive index as glass. Therefore, it improves the quality of the image. If immersion oil is not used, the image appears blurred or hazy.
Mechanical stage
- The mechanical stage holds the slide and allows it to be moved to the left, right, forward or backward by rotating the knobs.
- It is fitted with fine vernier graduations as on a ruler. This helps in relocating a specific field of examination.
Condenser
- The condenser illuminates the specimen and controls the amount of light and contrast. There are different types of ondensers. Some condensers have a rack-and pinion mechanism for up-and-down adjustment.
- The NA of a condenser should be equal to or greater than that of the objective with maximum NA.
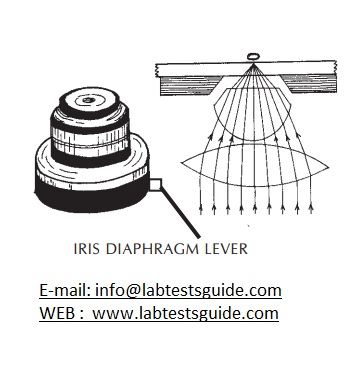
- An iris diaphragm is provided below the condenser. This adjusts the NA of the condenser when using objectives having low magnifying power.
- A swing-out type filter holder may be fitted above or under the condenser. In some microscopes the filter holder may not be swing-out type. The filter holder holds detachable filters when required.
- Condenser centring screws, when present, are used to align the condenser with the objective.
- A condenser raising knob may be present (if centring screws are not there), or the distance may be fixed.
Two-sided mirror
A mirror is the simplest illuminator. The two-sided mirror provides necessary illumination through reflection of natural or artificial light. It has two surfaces, one plain for artificial light and other concave for natural light. It is supported on two sides by a fork fixed on a mount in a way that permits free rotation.
A mirror is usually fitted on a mount or at the base of the microscope.
Built-in light sources
An illuminator is built into the base of the microscope. A halogen bulb provides the best illumination. On top of the illuminator is an in-built filter holder to fit the filter of desired quality.
Filters
- Blue filters are used to change the light from ordinary electric bulbs into a more natural white light.
- Neutral density filters are used to reduce brightness without changing the colour of the background.
- Green filters may be useful in some situations.
The object of AFB (Ziehl Neelsen) microscopy is to find AFB, which are stained red by carbol fuchsin. The intensity of the red colour decreases when blue/green filters are used. Blue/green filters are, therefore, not recommended for Ziehl Neelsen microscopy.
Immersion oil
- Immersion oil must be used with objectives having NA more than 1.0. This increases the resolving power of the objective.
- An immersion oil of medium viscosity and refractive index of 1.5 is adequate. Any synthetic non-drying oil with a refractive index of 1.5 and/or as recommended by the manufacturer should be used.
Halogen lamp
Halogen lamps are low wattage, high intensity lamps and are the preferred light source. Though costlier, these have the following advantages over tungsten lamps:
- emit white light
- have higher luminosity (brighter)
- have compact filament
- have longer life.
Routine Operation of the Microscope
- Ensure that the voltage supply in the laboratory corresponds to that permitted for the microscope; use a voltage protection device, if necessary
- Turn on the light source of the microscope
- With the light intensity knob, decrease the light while using the low magnification objective.
- Place a specimen slide on the stage. Make sure the slide is not placed upside down. Secure the slide to the slide holder of the mechanical stage
- Rotate the nose-piece to the 10x objective, and raise the stage to its maximum .
- Move the stage with the adjustment knobs to bring the desired section of the slide into the field of view.
- Focus the specimen under 10x objective using the coarse focusing knob and lowering the stage .
- Make sure the condenser is almost at its top position. Centre the condenser using condenser centring screws if these are provided in the microscope. For this take out one eye-piece and while looking down the tube, close the iris diaphragm till only a pin-hole remains. Check if this is located in the centre of the tube.
- Open the condenser iris diaphragm to 70%80% to adjust the contrast so that the field is evenly lighted .
- Many modern microscopes have pre-centred and fixed condensers. In these no adjustments are required.
- Adjust the interpupillary distance till the right and left images become one
- Focus the image with the right eye by looking into the right eye-piece and turning the focusing knob .
- Focus the image with the left eye by looking into the left eye-piece by turning the diopter ring .
- Put one drop of immersion oil on the specimen.
- Change to 100x objective.
- Increase the light by turning the intensity knob until a bright but comfortable illumination is achieved.
- Focus the specimen by turning the fine focusing knob.
- When the reading/observation has been recorded, rotate the objective away from the slide.
- Release the tension of the slide holder, and remove the slide.
- If immersion oil was used, wipe it from the objective with lens paper or muslin cloth at the end of each session of use. In general, avoid wiping the objective except when it seems to be dirty
- Turn off the light.
RELATED POSTS
View all