Ninhydrin is a chemical that detects ammonia and amines (primary and secondary). Once ninhydrin reacts with these chemicals, it produces an intense blue or purple color: Ruhemann purple. Ninhydrin is also the same chemical used to detect fingerprints.

It is effective because the terminal amines of a lysine residue in proteins and peptides were released on fingerprints and reacted with said chemical.
What is the ninhydrin test for?
- It plays an important role in monitoring deprotection in solid phase peptide synthesis. If nitrogen is deprotected, the ninhydrin test turns blue.
- It is used in the analysis of amino acids in proteins. Most amino acids are hydrolyzed and react with ninhydrin with the exception of proline. Some amino acid chains degrade. Therefore, a separate analysis is needed to identify amino acids that may react or not react with ninhydrin.
- It is used to verify a solution suspected of having ammonium ions. A treatment with ninhydrin would result in a dramatic purple color.
- It is usually used by forensic investigators in the analysis of fingerprints on porous surfaces. A finger mark containing amino acids is treated with a ninhydrin solution, which results in a purple amino acid finger crest pattern. Therefore, making the fingerprint visible.
What is the principle of the ninhydrin test?
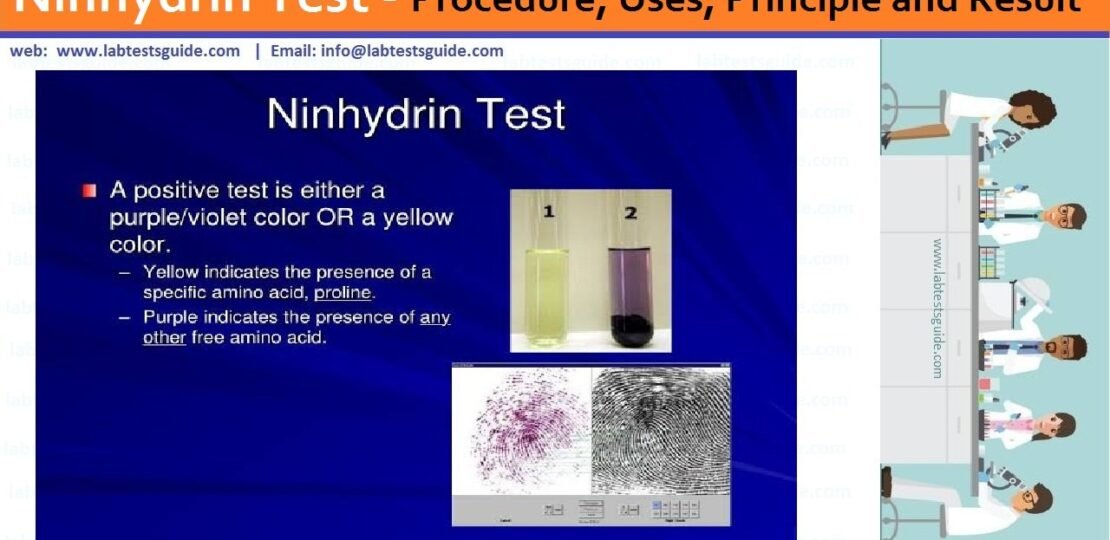
A ninhydrin test is a general test performed by all amino acids. The test is performed as a result of the reaction between the amino group of the free amino acid and ninhydrin. As you know, ninhydrin is a strong oxidizing agent. Its presence causes the amino acid to pass through oxidative deamination releasing ammonia and reduces the formation of ninhydrin.
The NH3 formed reacts with the ninhydrin molecule resulting in the formation of a blue substance. However, some amino acids such as proline and hydroxyproline do not lead to the production of blue or purple substances. They usually yield to a brown product.
What are the requirements for the ninhydrin test?
- Test solution which consists of 1% proline, alanine, and asparagines
- Water bath
- 2% ninhydrin in acetone
- dry test tubes
- Micro pipettes
How does the ninhydrin test work?
- A 0.2 gm of ninhydrin should be dissolved in 10 ml of acetone. In the absence of acetone, you can use ethanol.
- Mix 1% of amino acid solution in distilled water.
- An equivalent number of the test solution and distilled water, both 1 ml is put in a dry test tube. One test tube for the test solution and 1 test tube with distilled water as a control.
- Both test tubes should be poured with a few drops of 2% ninhydrin.
- Place the two test tubes in water bath for approximately five minutes.
- Watch for any signs of changes in color – blue or violet.
Note : Proline and hydroxyproline do not cause blue or violet discoloration. Instead, they produce color yellow. On the other hand, asparagine would result in brown color.
Why is Ninhydrin used in chromatography?
To detect amino acids in thin posterior chromatographic plates, several types of reagents are used and one of them is ninhydrin. It is the most preferred due to its high sensitivity. The resulting color remains blue or purple / violet with all amino acids, with the exception of proline and hydroxyproline, which causes the color to turn yellow. (2, 5 and 9)
Facts about the ninhydrin test
- Many bioanalytical procedures use ninhydrin, especially for the amino acid analysis method.
- The ninhydrin test is used by SSDs for the detection of residual protection in reusable surgical instruments.
- The amino acids react with ninhydrin, which causes discoloration.
- The ninhydrin test is used for both quantitative and qualitative purposes, such as chromatographic visualization and peptide sequencing. In addition, whenever you need research peptides for your work, you can always order from a licensed store that offers premium-quality research products.
- Bluish to purple discoloration is produced by amino acids a, while yellow to orange discoloration is caused by secondary amine such as proline.
- The ninhydrin test is extremely sensitive and can be used to visualize fingerprints.
- Ninhydrin is the most preferred chemical for the visualization of fingerprints in porous materials and paper, since it reacts with the amino acids in sweat that remain in a fingerprint.
- The strong compound formed by ninhydrin is called Ruhemann purpura.
- The strongly colored compound that is then formed is called Ruhemann purple.
- Ninhydrin reacts with compounds that contain amine, such as proteins in the blood.
Related Articles:
RELATED POSTS
View all
