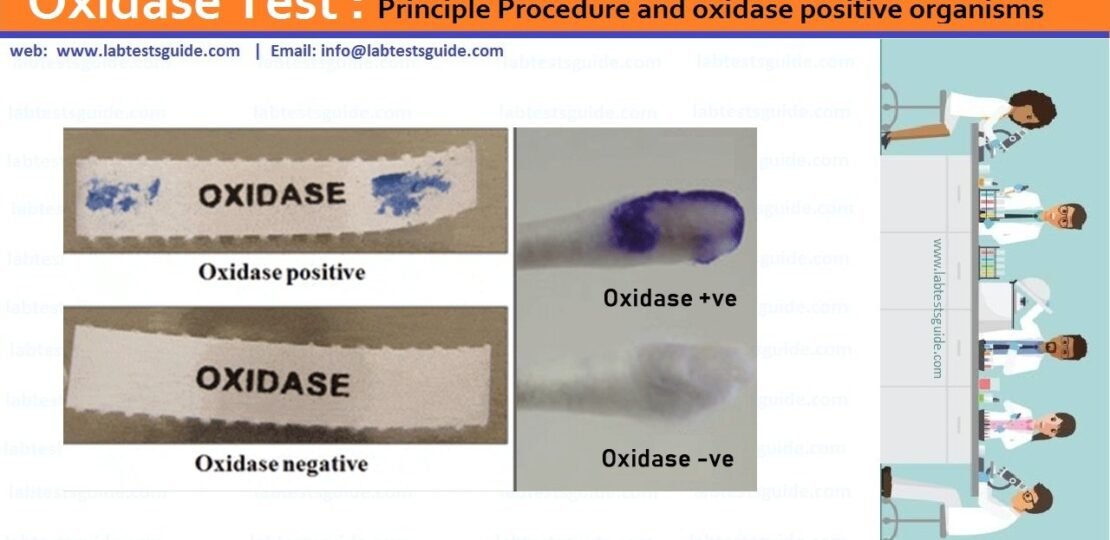
The oxidase test is used to identify bacteria that produce cytochrome c oxidase, an enzyme of the bacterial electron transport chain. When present, the cytochrome c oxidase oxidizes the reagent (tetramethyl-p-phenylenediamine) to (indophenols) purple color end product. When the enzyme is not present, the reagent remains reduced and is colorless.

Purpose of Oxidase test
Oxidase test is most helpful in screening colonies suspected of being one of the Enterobacteriaceae (all negative) and in identifying colonies suspected of belonging to other genera such as Aeromonas, Pseudomonas, Neisseria, Campylobacter, and Pasteurella (positive).
Test requirements for Oxidase test: Moist filter paper with the substrate (1% tetramethyl-p-phenylenediamine dihydrochloride), or commercially prepared paper disk, wooden wire or platinum wire.
Expected results of Oxidase test
Positive: Development of dark purple color (indophenols) within 10 seconds
Negative: Absence of color
Quality Control of Oxidase Test
Bacterial species showing positive and negative reactions should be run as controls at frequent intervals. The following are suggested:
A. Positive control: Pseudomonas aeruginosa
B. Negative control: Escherichia coli
Procedure of Oxidase test:
Oxidase test can be done using various methods. We will discuss the commonly used methods below.
- Dry filter paper – This is the most convenient method. Procedures are as follows:
- Soak the filter paper (Whatman’s # 1) in a 1% tertramethyl-p-phenylene-diamine dihydrochloride solution.
- Drain for about half a minute.
- Freeze dry the filter paper and store in a bottle; preferably the dark one and with a cap secured by screws.
- If used, it is a must to remove the strip, use distilled water to moisten the strip and put in the Petri dish.
- Using the loop (platinum), pick the colony and smear over the moist section.
- Wet Filter Paper – It is called the wet filter paper method because the filter is soaked in a 1% solution of the reagent. Using a loop (made from platinum material), rub the culture on the filter paper.
- Direct Plate – Add a reagent; ideally two to three drops to the suspected colonies on the agar plate. Just add enough reagent as putting too much reagent can alter the result. Watch out for any changes in color in a span of 10 seconds. This type of oxidase test should be done on the agar plate (non-selective).
- Swab method – the swab should be dip into the reagent and should be in contact with the suspected isolated colony. Watch out for any changes in color in 10 seconds.
- Test tube – Fresh bacteria culture is grown in a nutrient broth (4.5 ml). Growing should be done within 18 to 24 hours. Add Gaby and Hadley reagent (0.2ml). Shake the test tube to make sure the mixture is thoroughly mixed and the culture has thorough oxygenation. Watch out for any changes in color.
Precaution to be taken while performing oxidase test:
- Do not use Nickel-base alloy wires containing chromium and iron (nichrome) to pick the colony and make smear as this may give false positive results
- Interpret the results within 10 seconds, timing is critical
Name of Oxidase positive bacteria are: Mneomoics for Oxidase Positive Organisms- PVNCH (It’s just an acronym inspired by the famous mneomonic for Urease Positive organisms-PUNCH)
- P: Pseudomonas spp
- V: Vibrio cholerae
- N: Neisseria spp
- C: Campylobacter spp
- H: Helicobacter spp/ Haemophilus spp.
- Aeromonas spp
- Alcaligens
Related Articles:
RELATED POSTS
View all